Abstract
Background: The optimal treatment for severe venous thrombotic events (VTE) in children has not been determined and VTE recurrence and post thrombotic syndrome pose persistent clinical dilemmas. Growth arrest-specific 6 (GAS6), a vitamin K dependent γ-carboxylated protein, has been shown to enhance platelet activation and thrombus stabilization in murine models. Additionally, elevated levels of GAS6 have been reported in humans suffering from inflammatory conditions (systemic lupus erythematosus, sepsis, multiple sclerosis, etc.). Many of these same inflammatory conditions have been associated with development of venous thromboembolism (VTE) in multiple studies of risk factors, and children often develop VTE in the setting of inflammatory processes such as infection, cancer, and antiphospholipid antibodies. However, the role of inflammation in the initiation or progression of VTE has not been fully elucidated. A correlation has been demonstrated between elevated GAS6 levels and VTE risk in adults, but no similar studies have been done in the pediatric population or combined GAS6 with inflammatory markers. We present preliminary data on GAS6, its soluble transmembrane receptor AXL, and inflammatory cytokines in childhood VTE.
Methods: Children were prospectively enrolled into an IRB-approved inceptional cohort of thrombosis and thrombophilia. The study collected clinical data regarding risk factors, treatments, and outcomes, and a specimen repository stored serial, plasma and DNA samples. For this analysis, pediatric plasma samples collected within 10 days of an acute VTE were used to measure cytokines IL-6, IL-8, TGF β1, Galectin 3, and TNF-α using a luminex system. The platelet ligand GAS6 and its soluble transmembrane receptor AXL were also measured using an R&D Systems ELISA kit. Healthy volunteers with no history of a bleeding or clotting disorder, and on no medications were recruited for control samples. Data was expressed as median +/- interquartile range (IQR) and evaluated using Mann-Whitney rank sum test.
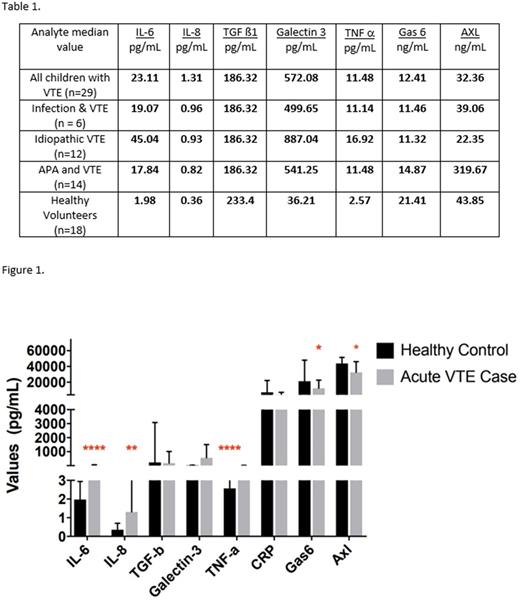
Results: Healthy controls consisted of 19 children and young adults with a mean age of 9.4 (SD 4.4, median 9.2). Twenty nine participants with acute thrombosis were included in this study. Clinically, the pediatric patients were 48% male, and had a mean age of 14.1 years (SD 4.54, median 15). Risk factors included: underlying medical condition 52%, idiopathic presentation 48%, antiphospholipid antibodies 48%, genetic thrombophilia 28%, familial thrombosis 21%, and vascular malformation 7%. Outcomes included VTE recurrence in 17% and post thrombotic syndrome in 14%. On the day of research blood sampling (days 0-10 of acute presentation), factor VIII activity was elevated in 66% with a median value of 213% in those elevated; D-dimer was elevated in 66% with a median value of 2240 ng/mL in those elevated. Compared to healthy volunteers, pediatric VTE subjects demonstrated higher median (±IQR) levels of IL-6 (23.11±53.24 pg/mL, n=29 vs. 1.98±1.92 pg/mL, n=18 p<0.0001), IL-8 (1.31±2.33 pg/mL, n=29 vs. 0.36±0.5 pg/mL, n=18 p<0.005), Galectin-3 (572.08±1355 pg/mL, n=29 vs. 36.21±7.28 pg/mL p<0.05), and TNF-α (11.48±18.62 pg/mL, n=29 vs. 2.57±1.125 pg/mL n=19 p<0.0001) (Table 1). There was no difference among groups of children with VTE related to infection, antiphospholipid antibodies or idiopathic presentation (Table 1). Unexpectedly, pediatric subjects demonstrated lower median levels of GAS6 (11.9±11.3 ng/mL, n=20 vs. 21.4±32.6 ng/mL, n=15 p<0.05) and AXL (32.36±17.9 ng/mL, n=29 vs. 43.85±20.5 ng/mL, n=16 p<0.05) (Table 1).
Discussion: In this pilot study, children with thrombosis expressed elevated levels of IL-6, IL-8, Galectin-3, and TNF-α, consistent with the finding of increased VTE in inflammatory states. Conversely, both GAS6 and soluble AXL were decreased in children with thrombosis. This decrease in GAS6 levels may be due to increased binding of GAS6 to AXL bound to platelets, pulling it out of plasma. Further research on the cytokine profile of VTE in children is warranted to understand the pathophysiology of this disorder and to plan rational, precision-medicine therapies.
Ng: Shire: Consultancy; CSL Behring Heimburger Award: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal